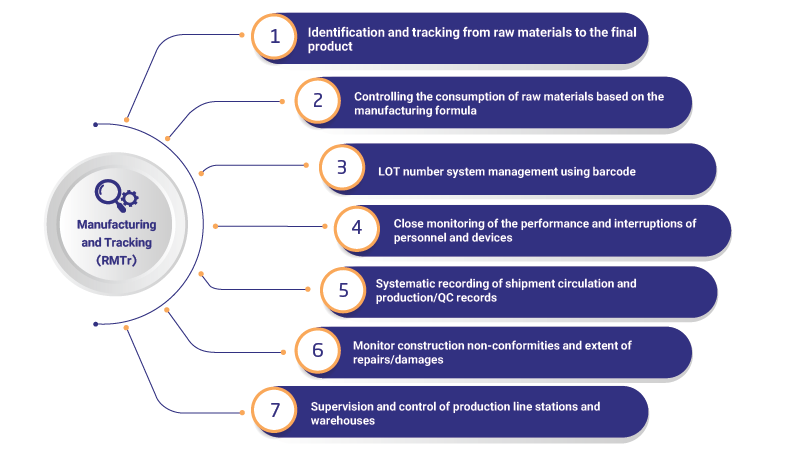

Raybod manufacturing and tracing (RMTR)
Identification and tracing is one of the most important topics in the production of health-oriented products and quality management systems, including ISO 13485 and GMP requirements; Electronic batch records (EBR-Electronic batch records) document the records of a product produced or being produced (in such a way that it is possible to understand what semi-manufactures each shipment (batch/ship) is made of, what materials each semi-manufacture is made of primary was created, who is the supplier of raw materials, who produced or controlled/tested each stage of production or QC, with what equipment, on what date and under what conditions, the values of each of the control/laboratory parameters of raw materials or what was the semi-finished product or product, which items were modified/reworked/reprocessed or scrapped, compliance of the shipment with the manufacturing formula (BOM) and the production process and dozens of other data and information that are important to the manufacturing company and regulatory and supervisory bodies has). The electronic batch record is actually the systematic implementation of the implementation method of identification and tracing, which is the most important feature of Raybod manufacturing and tracing operation software
Manufacturing operations and tracking
In the production of health-oriented products, including medical equipment and pharmaceutical products, product tracking from raw materials to the final product is very important. To help this complex process, Raibad's production management and tracking software is designed as the most specialized production and tracking software in the country, which is a powerful and comprehensive tool for managing production processes, quality control and ensuring the quality of the organization. By providing advanced and practical features, this software helps manufacturing companies to manage their production and tracking processes accurately and efficiently and to easily pass the requirements of regulatory bodies, including the General Directorate of Medical Equipment and the Food and Drug Administration. Key features of this software: 1. Traceability of raw materials: the ability to track all raw materials from the moment they enter the factory until they are used in the production line 2. Inventory control: detailed inventory management of warehouses, raw materials, semi-finished products, and finished products. 3. Production planning: providing advanced subsystems and tools for production planning and scheduling, with the ability to adjust and optimize the production line. 4. Quality control: Providing quality control sub-systems for checking and monitoring quality at every stage of production. 5. Data reporting and analysis: the possibility of preparing detailed reports and data analysis for continuous improvement of processes and intelligent decision making. 6. Warning and notification system: intelligent warnings to announce critical cases and prevent potential problems from occurring
Features and facilities
The possibility of introducing production stages with the ability to group and determine the nature of the stage (operations, control, storage, etc.)
Introduction of goods (raw materials, parts and products) with the possibility of inserting photos, GTIN, IRC and…
Creating a unique group identifier (LOT/Batch/Serial number) with a maximum length of 20 digits
Introducing the manufacturing formula (BOM) with versioning capability
Introduction of OPC with the possibility of connecting SOP, map number, etc. for each step with versioning capability
The possibility of defining control parameters during the process/after the process
The possibility of introducing production halls, location codes and machines
Defining the timing of stages according to different machines for one stage and determining the basis of the number and standard time for each stage
The possibility of printing the cargo travel sheet/cargo travel label with the selected content to stick on the cargo
The possibility of using linear and QR barcode scanners in all stages of production, quality control, branding and packaging to prevent human errors.
The possibility of real-time reporting of the items in the production line by part, stage and operator; The items in the correctional and waste warehouse are divided into parts and stages
Ability to report on the performance of each production line operator, in a selectable time period, including all worked items, items that have been modified, items that have been damaged, items that have been handed over to the operator but not returned.
Performance report of quality control operator, production operator and external contractor
The possibility of recording 100% or samples of control parameters by quality control personnel or production operators by computer/tablet/mobile phone
Ability to design and print product labels, product labels in selected formats
Work station inventory report and cargo circulation report between production and control stations
Cargo management from raw materials to the final product with automatic lot number mechanism and the possibility of receiving a tracking identification report
Managing users and determining the access levels of people to different departments and the ability to limit operations
save expense
1- Reducing the cost of quality related to Batch records management
2- Reducing the cost associated with the consumption of raw materials and semi-finished and product waste
3- Reducing costs related to human resources
The operation module of production and identification of ribbed tracking makes the processes simple, intelligent and automatic, and by taking advantage of advanced features and capabilities, it guarantees efficient and error-free operations.
If you are the CEO, you don’t need to worry about whether the raw materials delivered to the operator (which in many cases are expensive) have been fully converted to parts/semi-finished and delivered to the warehouse? Are the shipments that are delivered to the operator or contractor missing or missing? What is the operator’s monthly, quarterly or annual statistics regarding its performance? You don’t need to worry about the audit and license of your production anymore, because all the processes are controlled by the system and if there is any disturbance, the warning will be displayed for your experts and personnel. If the time of your human resources is spent on warehouse management, filling out forms, keeping Excel files up to date, collecting data from different departments, there is a software that automates all of this and you can spend your time in the department. Use other employees of the organization and get the most out of the salary you pay him. All that said is not too far fetched, all of this is accomplished with Rybad’s production operations and tracking identification software.
If you are a technical manager or quality assurance manager, imagine not having to worry about filling out forms related to warehouse, production, quality control/laboratory, etc. You don’t have to worry about the contents printed on the label and packaging of the product. You don’t need to collect data to create reports because everything you need is already in the system. You no longer need to maintain and update Excel files, forms, etc. Whenever you need to track a semi-finished product or part (such as when you have an external audit), you can instantly enter the desired LOT/Batch number in the system and the tracking report of that raw material/semi-finished product/product including the values Get control/laboratory parameters and other attachments. This utopia is not far off, all made possible by Rybad’s production operations and tracking software.
In companies that produce health products, including medical devices, pharmaceutical products, cosmetics, and food, you can manage batch records management processes with Raibod’s production operations and tracking identification software. Optimize yourself, achieve higher quality standards and achieve significant cost savings in your operations.
