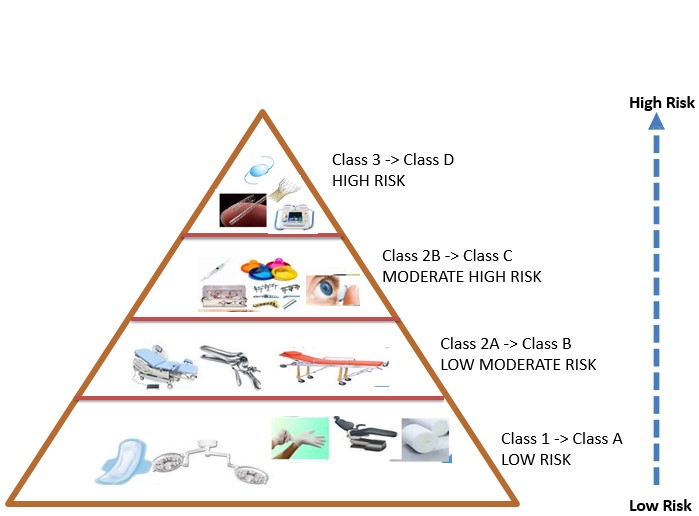
Medical device hazard class
The term medical devices has different definitions in different countries, but in general, any device, tool, or equipment that is manufactured by a manufacturer for humans with the aim of preventing, diagnosing, treating, or reducing the pain of a disease is called medical equipment. Medical equipment, like drugs and other health technologies, is essential for the care and treatment of patients. Quality, safety, and health and treatment depend on the safety and quality of medical equipment. According to the experiences of developed countries, the first step in creating such laws is to classify medical equipment based on their risk and develop criteria based on this classification and appropriate for each category of equipment, which has led to the creation of many criteria for measuring the quality and safety of medical equipment.

Quality management system
1402/01/01 / the author
One of the most widely used standards that is popular all over the world…
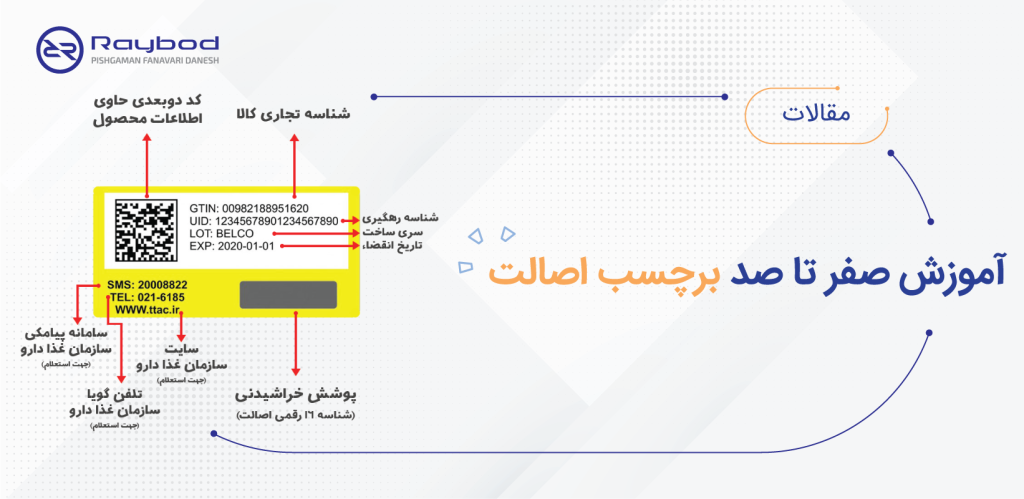
Zero to one hundred product authenticity label training
1403/03/04 / Engineer Masoud Abiyar
This is a complete educational article for product authenticity label
In Article 10 of the Medical Devices Regulation, all natural and legal persons are required to comply with the criteria for classifying medical devices and be aware of the risks of the relevant devices, and to act in the field of production, import, distribution and after-sales service of medical devices.
Keywords: Hazard class, risk level, medical devices, medical device production management, Raybod
Hazard class determines the safety and effectiveness of medical products and is determined based on the following characteristics:
1. Duration of contact of the device with the patient (transient, short-term, long-term)
2. Invasiveness
3. Degree of invasiveness (through body cavities or surgically)
4. Anatomy involved in the process of functioning of the medical device (such as the central nervous system)
5. Active or passive
6. Special conditions (such as containing pharmaceuticals or animal tissue)
7. Level of risk posed to the body
Corrective and preventive measures
1402/01/01 / the author
Corrective and preventive measures as the most important part of quality management…

